


1. Why use quality control for real-time PCR?
Due to the problems of reagent/equipment instability, low nucleic acid extraction efficiency, and sample cross-contamination in the entire process of sample processing, nucleic acid extraction, and PCR amplification, a quality control system needs to be set up to monitor the entire process of nucleic acid extraction and PCR amplification. The quality control system can reduce experimental errors, ensure the accuracy of sample testing, and avoid false negatives and false positives during the testing process.
2. Composition of quality control
Positive control (PC): Monitoring system failure, generally a plasmid containing the target component or fragment.
Negative control (NC): monitor the contamination of the reaction system, generally a plasmid that does not contain the target component or fragment.
No template control (NTC): monitor the contamination of the reaction system, generally deionized water.
Internal standard (internal reference): to calibrate biological errors to determine the effectiveness of nucleic acid extraction and amplification efficiency.
Repeat the experiment: reduce the remaining errors, generally more than 2-3 times.
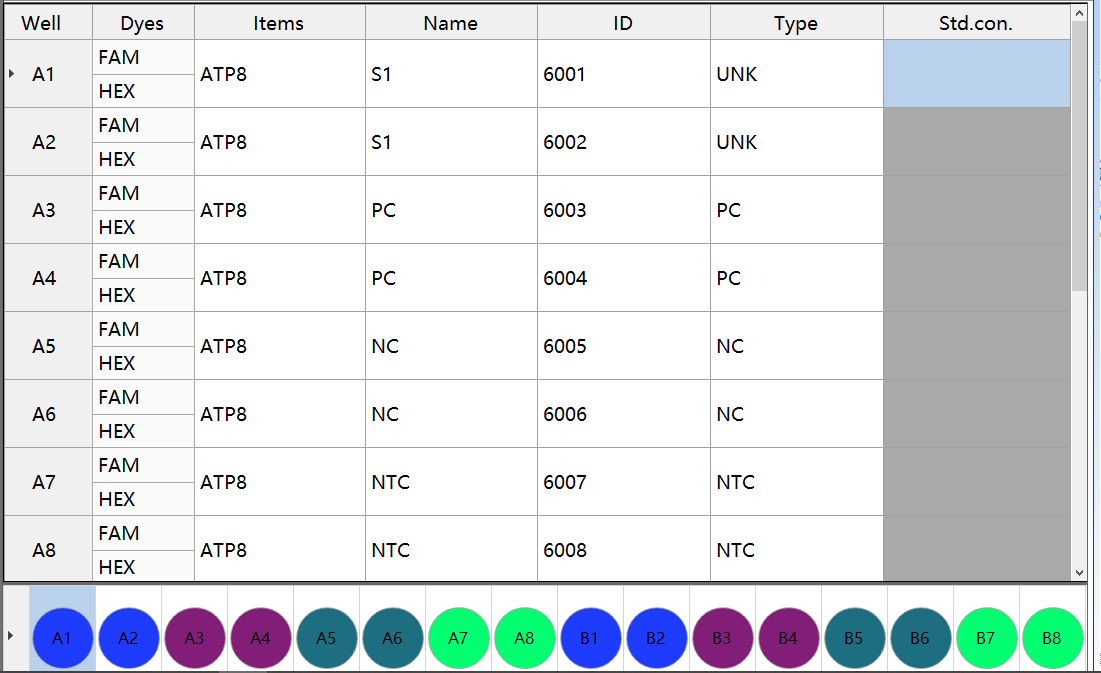
GB∕T38164-2019 Animal-derived component detection method quality control product setting example
The method for detecting the internal reference gene 18SrRNA in the animal-derived detection operation can be set in the second channel. Single-channel products can be carried out in block B, and the setting is the same as block A
3. Quality control system setting requirements and process control
Generally, experiments without special requirements need to set up negative and positive controls, such as African swine fever virus detection (TCVMA 5-2018), food-borne pathogen detection(SNT1870-2016) and etc. For strict experiments, internal references must be set and carried out 2 -3 parallel experiments, such as animal-derived testing (GB∕T38164-2019), transgenic quantitative experiments (GBT19495.5-2018).
For clinical diagnosis involving human samples, in addition to the quality control settings, quality control of the entire nucleic acid extraction and PCR amplification process is also required, Such as norovirus detection (GB 4789.42-2016), COVID-19 detection, etc.
In the actual experiment process, it is necessary to comprehensively consider the actual conditions such as the experiment environment, operation process, and experiment cost in order to select an appropriate method for quality control.
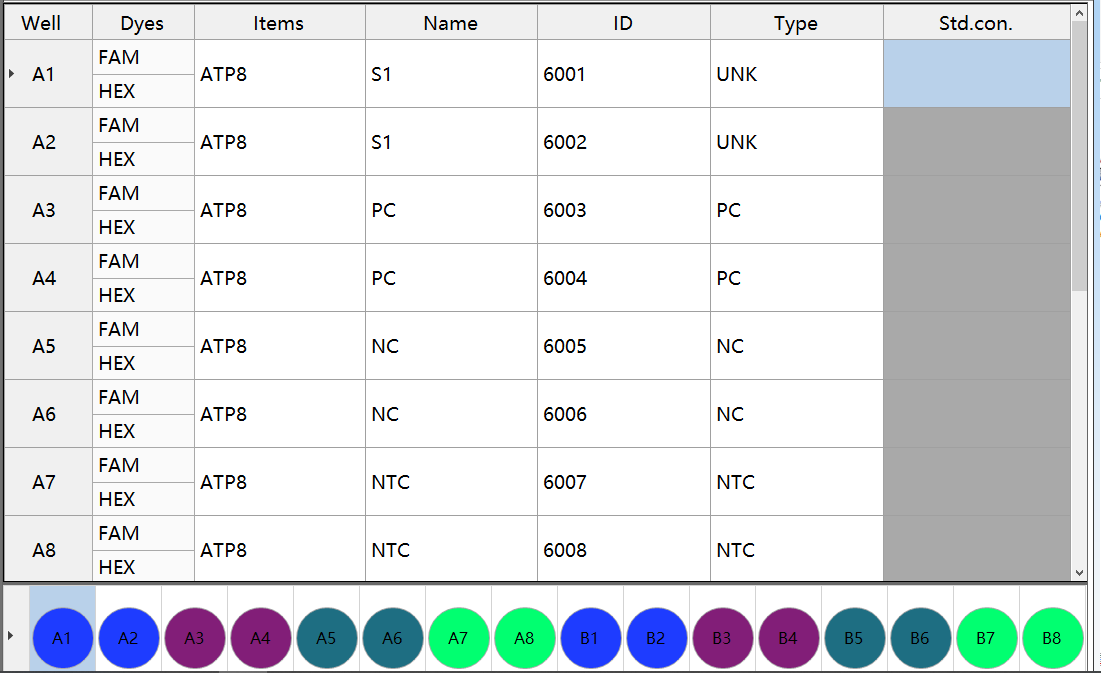
(1) Examples of amplification control (A5-A7)
GB 4789.42-2016 Norovirus test Amplification control example
Rule: Inhibition index <2.00, the amplification experiment is established, otherwise, the reaction is invalid.
Method: Inhibition index=A6Ct value-A5Ct value. If the condition is met, the Ct value of A1 hole is used as the result; If it is not satisfied, judge A7Ct value-A5Ct value, and use A2 hole Ct value as the result after satisfying the condition; If the inhibition index of both reaction holes is ≥2.00, the reaction is invalid. (If the reaction result is positive, it can be judged as positive if appropriate)
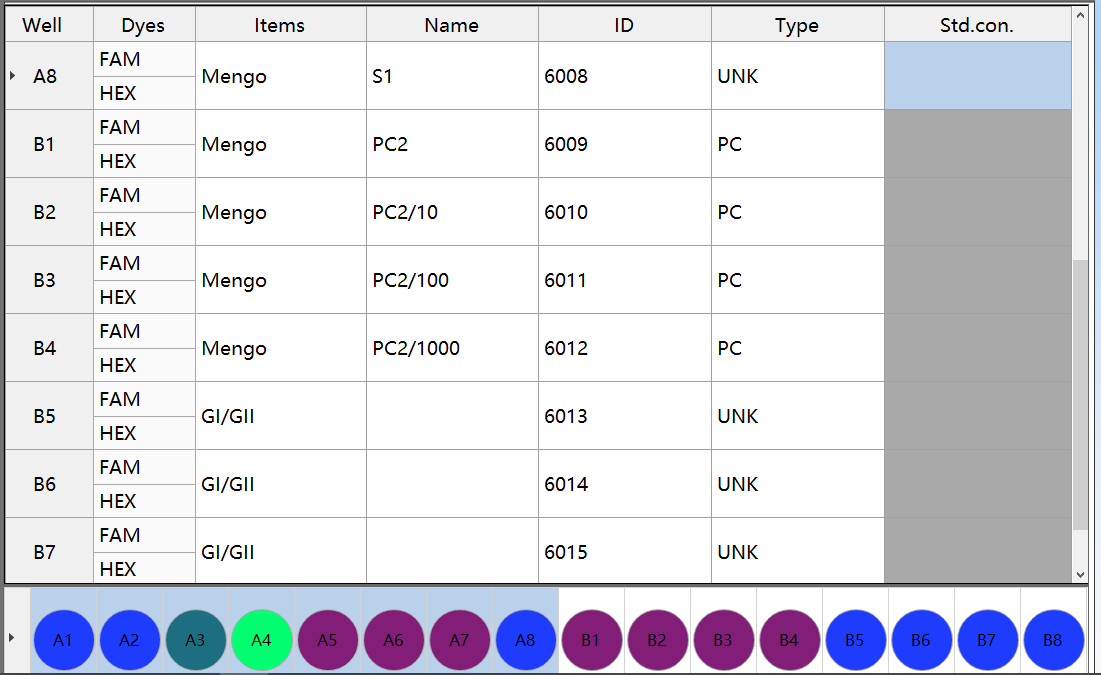
(2) Process control of nucleic acid extraction (A8-B4)
GB 4789.42-2016 Norovirus test Nucleic acid extraction process control example
Rule: If the nucleic acid extraction efficiency is ≥1%, the nucleic acid extraction is effective. If the extraction efficiency is less than 1%, it needs to be tested again (if the reaction result is positive, it can be judged as positive if appropriate)
Method: Establish a standard curve through wells B1-B4 (PC2 concentration is 1). Nucleic acid extraction efficiency = the concentration corresponding to the Ct value of well A8 × 100%.
4. Evaluation index of real-time PCR diagnostic test
(1) Authenticity evaluation
①: Sensitivity: also known as the true positive rate, that is, the probability of actually having a disease and being correctly judged as having a disease according to the diagnostic test.
②: Specificity: also known as the true negative rate, that is, the probability that the actual disease-free is correctly judged as disease-free by the diagnostic test.
③: False negative rate: also known as true positive rate, that is, the probability of a person who is actually sick but is determined to be non-ill according to the diagnostic test.
④: False positive rate: the probability that there is no actual disease but is determined to be diseased according to the diagnostic test.
(2) Reliability evaluation
①: Coefficient of variation: When used as a quantitative test, the coefficient of variation can be used to express reliability, that is, the ratio of the standard deviation of the measured average to the measured average.
②: Accordance rate: refers to the ratio of the number of people who have both positive and negative diagnosis results for the same batch of subjects in the total number of people undergoing diagnostic tests.